Surface Treatment of Gold Using Aqua Plasma®
Samco Inc.
Introduction
Gold, alongside copper and silver, serves as a widely utilized type of electrode in various applications. Despite its high resistance to oxidation, some recent studies [1][2] have demonstrated that exposure to oxygen plasma can lead to the formation of Au2O3 on the surface of gold. Furthermore, this oxidation decreases the electrical conductivity and impairs the bonding capabilities of gold.
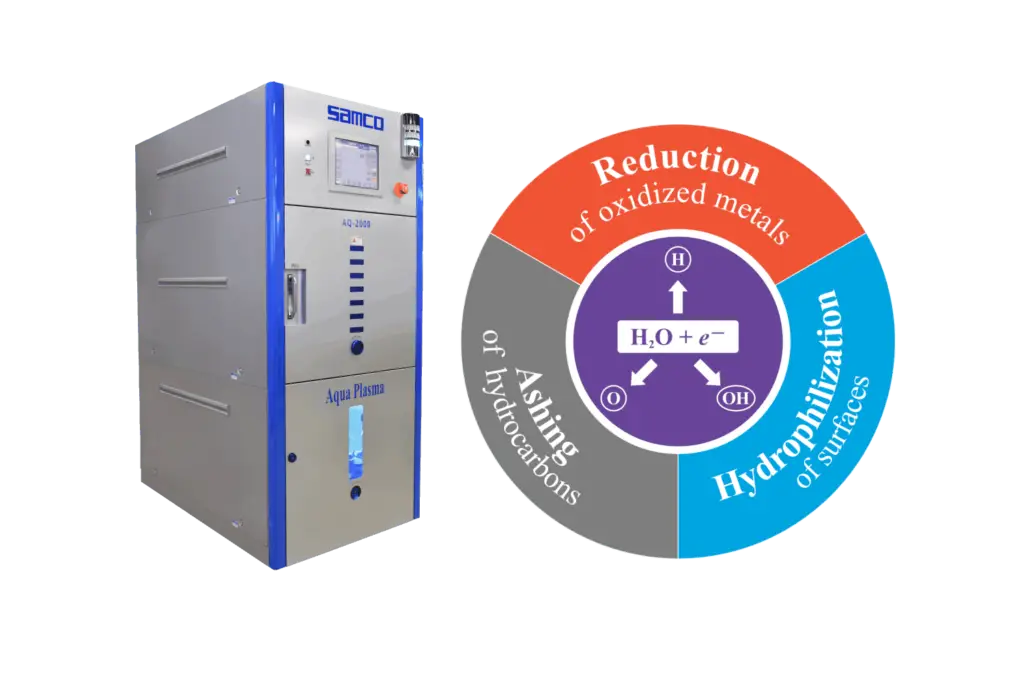
Aqua Plasma® technology generates H, OH, and O radicals in the plasma environment. These radicals facilitate chemical reactions that effectively reduce oxidized copper and silver, hydrophilize the surface, and remove the hydrocarbons with oxidation (Figure 1). Consequently, Aqua Plasma® has found extensive applications in semiconductor manufacturing processes, ranging from wafer processing to lead frame processing. Its uses include descum removal prior to plating, pre-bonding cleaning, and hydrophilization before molding [3][4].
This study investigates the oxidation of gold induced by oxygen plasma and the reduction effects achieved through Aqua Plasma® treatment.
Experiment
To assess the effect on gold electrodes, gold thin films with a thickness of 500 nm sputtered onto 4-inch silicon wafers were used. The Aqua Plasma® Cleaner AQ-2000 was employed for all plasma treatments. Samples were positioned on the ground electrode and processed without heating. Only the gas type was varied, while gas flow rate, process pressure, RF power, and processing duration remained constant. Three experimental conditions were evaluated: oxygen plasma treatment, Aqua Plasma® treatment, and sequential treatment of oxygen plasma followed by Aqua Plasma®. X-ray Photoelectron Spectroscopy (XPS) was used to analyze the oxidation state on the surface of the gold before and after treatment.
Results and Discussion
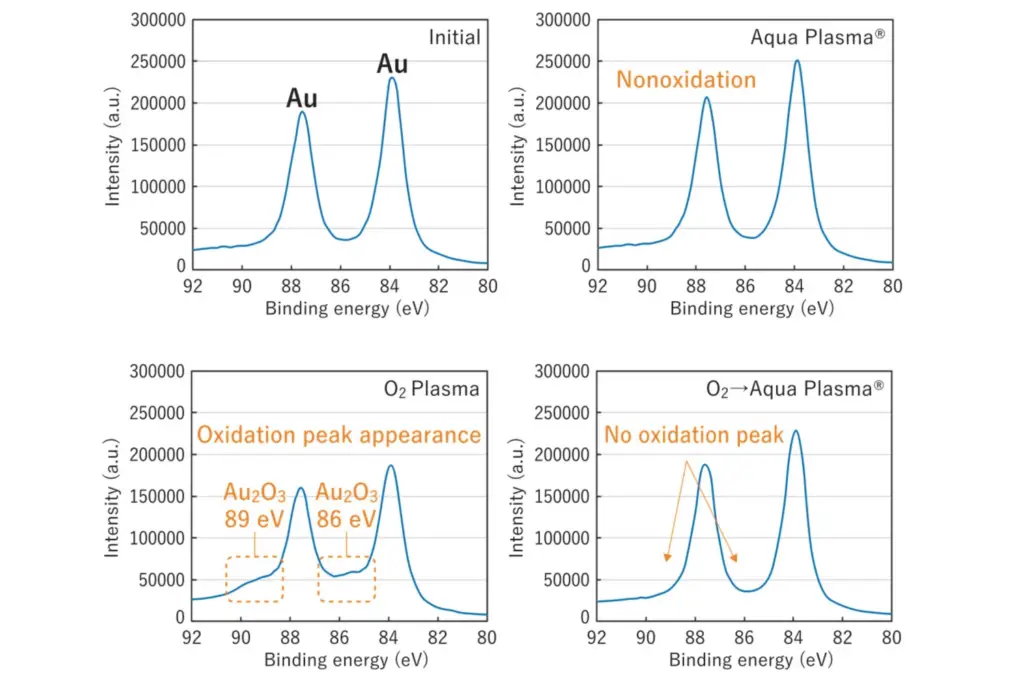
Visual changes on the surface of the gold were not observed after each plasma treatment. However, XPS analysis detected Au2O3 peaks in the oxygen plasma-treated samples (Figure 2). Notably, these peaks were absent in the Aqua Plasma®-treated samples. Moreover, when the Aqua Plasma® treatment was applied to the oxygen plasma-treated samples, the Au2O3 peaks disappeared. These findings substantiate both the oxidation prevention and the reduction capabilities of the Aqua Plasma® on gold surfaces.
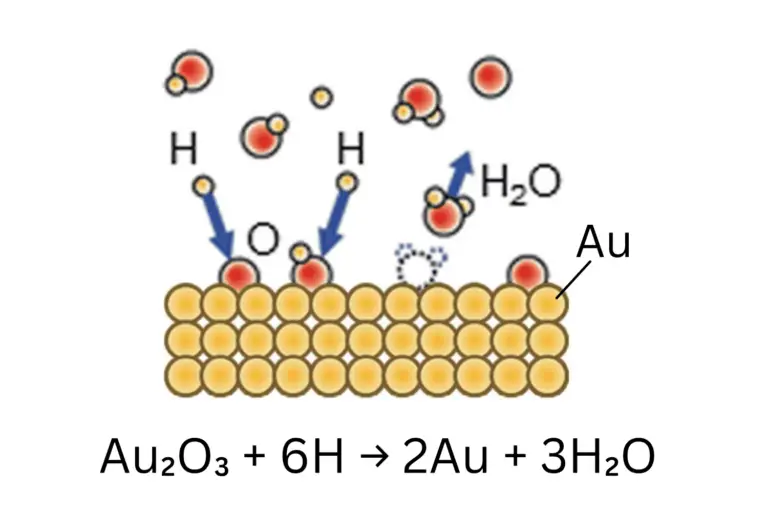
The proposed reduction mechanism involves H radicals present in Aqua Plasma®, analogous to the reduction process observed in oxidized copper and silver (Figure 3).
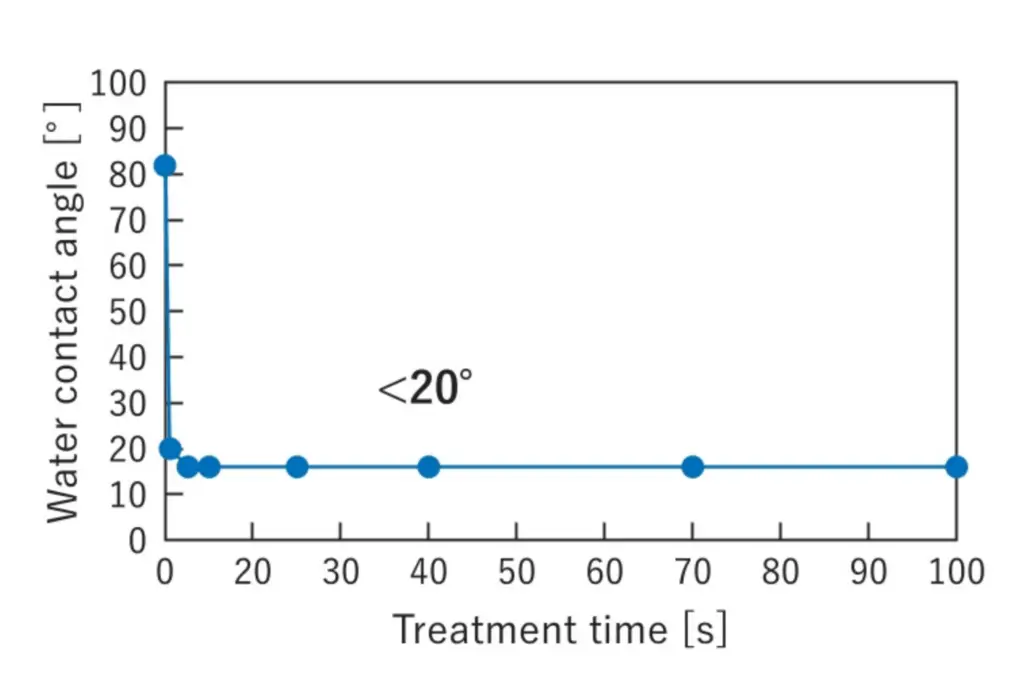
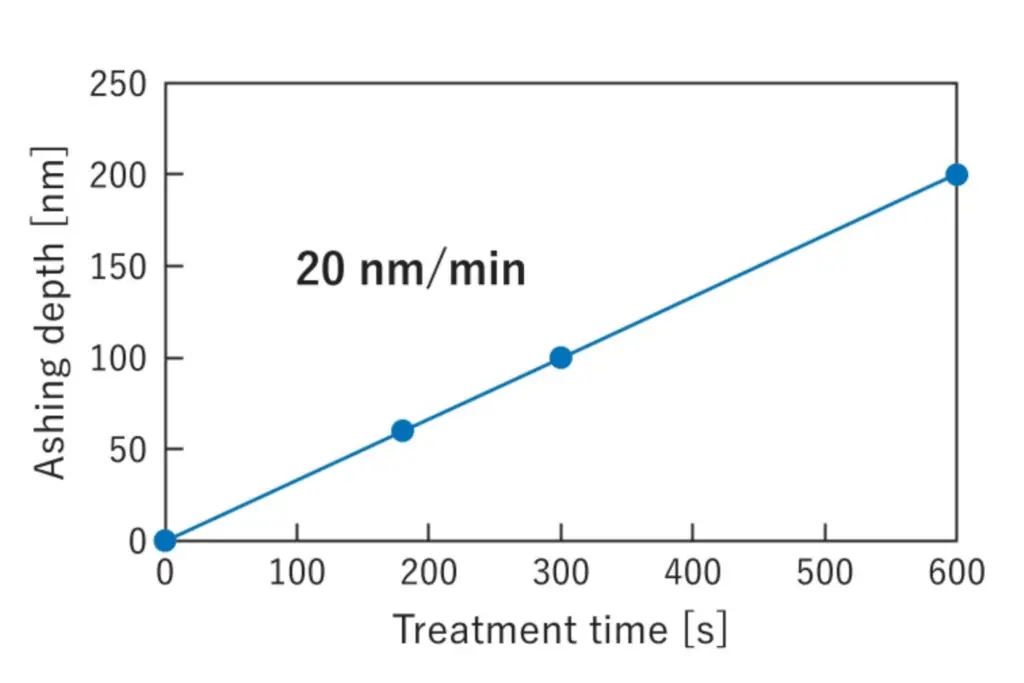
Concurrent with the reduction process, the surface of the gold exhibited enhanced hydrophilicity, with contact angles below 20° (Figure 4). Additionally, Aqua Plasma® demonstrated the ability to ash and remove hydrocarbons such as photoresist at a rate of approximately 20 nm/min (Figure 5).
The results of this study suggest that Aqua Plasma® offers a promising new approach for surface treatment of gold in semiconductor manufacturing processes. Its ability to prevent oxidation of the gold, reduce the gold surface oxides, and enhance surface properties makes it a versatile tool for various applications in the industry.
For those interested in exploring the potential of Aqua Plasma® technology, we invite you to contact our team for further information or to arrange a demonstration using production-scale equipment.
References
[1] H. Tsai et al., Surf Sci, vol. 537, no. 1–3, 2003.
[2] M. Yamamoto et al., Micromachines, vol. 10, no. 2, 2019.
[3] SAMCO NOW Vol. 94, 96, 103, and 111
[4] H. Terai, et al. IEEJ Trans. Sensors Micromachines 139, 157–162 (2019). 11–14, 2019.
[5] M. Tsumaya et al. 71st Spring Meeting of the Japan Society of Applied Physics, 24a-61B-2, 2024. (in Japanese)


